Photo credits: ScenTree SAS
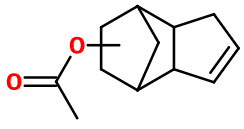
Cyclacet
Green > Juicy Fruits > Anisic > Lavender > Terpenic
acetate de verdyl ; Verdyl acetate ; Herbaflorat ; Jasmacyclene ; Greenenyl acetate ; 3a,4,5,6,7,7a-hexahydro-4,7-methano-1H-indenyl acetate ; acetate de 3a,4,5,6,7,7a-hexahydro-4,7-methano-1H-indenyl ; Tricyclo(5.2.1.02,6)dec-3-enyl acetate ; acetate de tricyclo(5.2.1.02,6)dec-3-enyl

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
Cyclacet - 30gr | - |
Visit website
|
- | - | - |
General Presentation
-
CAS N° :
54830-99-8 -
EINECS number :
259-367-2 -
FEMA number :
Donnée indisponible. -
FLAVIS number :
Donnée indisponible.
-
JECFA number :
Donnée indisponible. -
Volatility :
Base -
Price Range :
€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
1,07 -
Refractive Index @20°C :
Data not available. -
Optical rotation :
Data not available. -
Vapor pressure :
Data not available. -
Flash Point :
111°C (231,8°F)
-
Molecular formula :
C12H16O2 -
Molecular Weight :
192,26 g/mol -
Log P :
3,9 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
247°C (476,6°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Cyclacet can be used in soap, detergent and air freshner bases for its stability, and to bring a quite functional green and aromatic note.
Year of discovery :
1959
Natural availability :
Cyclacet is not found in nature. Thus, it is not extracted from any plant.
Isomerism :
Cyclacet is made of two isomers, formed during its synthesis. Their smell being not so different (one of the isomers is more woody and coniferous anyway), a mixture of the two isomers is used in perfumery.
Synthesis precursor :
Cyclacet is not used for the synthesis of another compound used in perfumes.
Synthesis route :
Cyclacet can be obtained with an addition reaction involving Acetic Acid and dicyclopentadiene, also using an acidic catalysor as concentrated sulfuric acid. This reaction gives birth to a mixture of two positional isomers, that are not separated afterwards.
Stability :
Esters may form their corresponding acid under the effect of heat.
Other comments :
Both imbricated cycles of the molecule are often associated with a woody note. It is the case for example in Mysore acetate, whose ester function is on the other side of the two cycles. This woody facet is anyway less percievable in Cyclacet.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment