Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
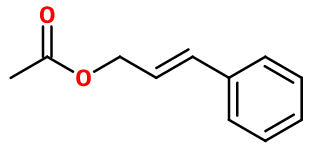
CINNAMYL ACETATE | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
103-54-8 -
EINECS number :
203-121-9 -
FEMA number :
2292 -
FLAVIS number :
09.018
-
JECFA number :
650 -
Volatility :
Heart -
Price Range :
€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
1,053 -
Refractive Index @20°C :
Data not available. -
Optical rotation :
Data not available. -
Vapor pressure :
Data not available. -
Flash Point :
118°C (244,4°F)
-
Molecular formula :
C11H12O2 -
Molecular Weight :
176,22 g/mol -
Log P :
2,9 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
264°C (507,2°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Cinnamyl acetate is used in reproductions of hyacinth, rosy and woody-spicy notes, to provide a green and balsamic effect.
Year of discovery :
Data not available.
Natural availability :
Cinnamyl acetate can be found in small quantity in some plant extracts such as Ylang-Ylang Extra EO (and other ylang fractions), Laurel EO and Cinnamon Leaf EO, as well as Narcissus Absolute, among others.
Isomerism :
Cinnamyl acetate has two diastereoisomers, due to the presence of a double bond in its structure. The (E) isomer of Cinnamyl acetate has an even sweeter smell than the (Z) isomer. Anyway, both have a similar smell, which explains the use of a racemic mixture of the two isomers in perfumery.
Synthesis precursor :
Cinnamyl acetate is not a precursor of the synthesis of another compound of olfactive interest.
Synthesis route :
Cinnamyl acetate is synthesized by an esterification reaction between Cinnamyl Alcohol and acetic acid. This reaction is catalyzed by the presence of a small quantity of a strong acid such as sulphuric acid. In addition, the yield of this reaction can be improved by the use of acetic anhydride or chloroacetic acid, rather than acetic acid.
Stability :
Esters my form their corresponding acid under the effect of heat.
Other comments :
Cinnamyl acetate is an ester obtained from Cinnamyl Alcohol, rather than cinnamic acid, as it is the case for compounds called Cinnamates.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















