Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
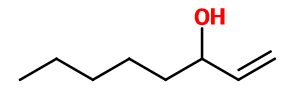
1-OCTEN-3-OL | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
3391-86-4 -
EINECS number :
222-226-0 -
FEMA number :
2805 -
FLAVIS number :
02.023
-
JECFA number :
1152 -
Volatility :
Heart -
Price Range :
€€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,83 - 0,85 -
Refractive Index @20°C :
1.435 - 1.439 -
Optical rotation :
Data not available. -
Vapor pressure :
Data not available. -
Flash Point :
63°C (145,4°F)
-
Molecular formula :
C8H16O -
Molecular Weight :
128,22 g/mol -
Log P :
2,7 -
Fusion Point :
-49°C (-56,2°F) -
Boiling Point :
180°C (356°F) -
Detection Threshold :
Seuil de détection : 14 ppb (0,0000014%)
Seuil de reconnaissance : 25 ppb (0,0000025%)
Chemistry & Uses
Uses in perfumery :
1,3-Octenol is used mainly in mushroom, lavender, woody and foody notes.
Year of discovery :
Data not available.
Natural availability :
The (R) enantiomer of 1,3-Octenol is present at 97% in the aromatic principle of mushrooms and 89% in the chanterelles aromatic principle. Nevertheless, 1,3-Octenol is not extracted in its natural state.
Isomerism :
The (R) enantiomer of 1,3-Octenol is more powerful than the (S) enantiomer, which has a vegetable smell. In perfumery, the mixture both isomers is the most used.
Synthesis precursor :
1,3-Octenol is a precursor for the synthesis of esters obtained by reaction between this alcohol and a carboxylic acid.
Synthesis route :
1,3-Octenol can be obtained by a Grignard reaction reacting vinylmagnesium bromide with hexanal.
Stability :
Stable in acidic products, but not so much in alkaline bleaches or detergents.
Other comments :
Data not available.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















