Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
CAS N° :
123-35-3 -
EINECS number :
204-622-5 -
FEMA number :
2762 -
FLAVIS number :
01.008
-
JECFA number :
1327 -
Volatility :
Head -
Price Range :
€€€
Physico chemical properties
-
Appearance :
Pale yellow liquid -
Density :
0,792 -
Refractive Index @20°C :
Data not available. -
Optical rotation :
Data not available. -
Vapor pressure :
Data not available. -
Flash Point :
50°C
-
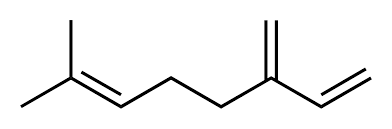
Molecular formula :
C10H16 -
Molecular Weight :
136,23 g/mol -
Log P :
5,29 -
Fusion Point :
<-80°C -
Boiling Point :
169°C -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Myrcene allows to change an apricot into a mango and to add a terpenic facet to floral notes.
Year of discovery :
Data not available.
Natural availability :
Myrcene is found in many plants. The main sources of natural Myrcene are Celery Leaf EO (up to 35%), Lemongrass EO (up to 15%) and Microstrobos fitzgeraldii leaf (up to 25%), found in Australia.
Isomerism :
Myrcene has many isomers with the same formula: D-Limonene, alpha-Pinene and beta-Pinene are part of it, but do not share the same smell.
Synthesis precursor :
Myrcene is a terpene precursor to the synthesis of many compounds of olfactory interest. It is an intermediate in the synthesis of Geraniol, Nerol and Linalool: an attack with hydrochloric acid followed by an esterification on the compound obtained allows to synthesize them. A protection of two alcene functions of this molecule allows to synthesize Myrcenol. It may be the starting point for the synthesis of Citronellal and Hydroxycitronellal. A reaction with diethylamine makes it a major precursor in the synthesis of L-Menthol. Finally, it is the precursor to the synthesis of Iso E Super® by a Diels-Alder reaction with 3-methyl-3-penten-2-one.
Synthesis route :
Myrcene is synthesized by a pyrolysis of beta-Pinene, which consists in heating this compound strongly (usually around 200°C) so that a particular bond breaks.
Stability :
Terpenes tend to polymerize by oxydation.
Very unstable in alkaline functional bases as soap. In this base, its smell in not percievable. Also unstable in candle bases.
Other comments :
Myrcene has a very singular note of caper, making a distinction with other terpenes.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

