Photo credits: ScenTree SAS
Florol®
Florosa® ; 4-methyl-2-(2-methylpropyl)oxan-4-ol ; 2-isobutyl-4-hydroxy-4-methyltetrahydropyran ; Floral pyranol ; Floriffol ; Florosol ; Florotyl ; Flowerol ; Frescoflor ; Keflorol 90 ; Muguetol ; Pyranol ; Tetrahydro-4-methyl-2-(2-methylpropyl)pyran-4-ol

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
FLOROL® | 966458 |
Visit website
|
Molecules |

|
- | - |
|
|
Florol - 30 Gr | - |
Visit website
|
- | - | - | |
|
|
Pyranol | 30221242 |
Visit website
|
Molecule | - | - | |
|
|
Pyranol BMBcert™ | 30770688 |
Visit website
|
Molecule | - | - |
General Presentation
-
CAS N° :
63500-71-0 -
EINECS number :
405-040-6 -
FEMA number :
Donnée indisponible. -
FLAVIS number :
Donnée indisponible.
-
JECFA number :
Donnée indisponible. -
Volatility :
Heart -
Price Range :
€
Physico chemical properties
-
Appearance :
Colorless liquid -
Density :
0,952 -
Refractive Index @20°C :
1,455 – 1,460 -
Optical rotation :
Data not available. -
Vapor pressure :
0,0486 hPa @20°C -
Flash Point :
>100°C (>212°F)
-
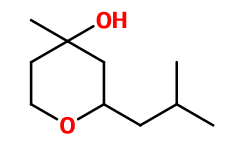
Molecular formula :
C10H20O2 -
Molecular Weight :
172,27 g/mol -
Log P :
2,22 -
Fusion Point :
<-100°C -
Boiling Point :
227°C -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Florol® is used to replace Hydroxycitronnellal, for regulatory reasons, for lily of the valley or lilac accords in particular. However, it is less fresh and floral-lily of the valley than Hydroxycitronellal. Also used for its stability.
Year of discovery :
Discovered in 1986. ''Florol® '' tradename has been published and protected by Firmenich SA since 18/02/1988 (brand N°521114)
Natural availability :
Florol® is not available in its natural state.
Isomerism :
The molecule has an asymmetric carbon, but it is the racemic mixture of Florol® that is used in perfumery. Hydroxycitronellal is a constitutional isomer of Florol®. Moreover, these two molecules are used for the same purpose in perfumery: often for reproductions of lily of the valley notes. In compositions, Florol® tends to replace Hydroxycitronellal as it is not regulated.
Synthesis precursor :
Florol® is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
The synthesis of Florol® is made by cyclocondensation between 3-methyl-3-buten-1-ol and 3-methylbutanal, on silica gel and aluminum oxide, and in the presence of solvent.
Stability :
Unstable in acidic products, except in fabric conditioners, and in alkaline products, except powder detergents.
Other comments :
Unlike Hydroxycitronellal, Florol® is not regulated or allergenic.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

