Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
Salicylate d'Amyle - 30gr | - |
Visit website
|
- | - | - |
General Presentation
-
CAS N° :
2050-08-0 -
EINECS number :
218-080-2 -
FEMA number :
Donnée indisponible. -
FLAVIS number :
09.762
-
JECFA number :
Donnée indisponible. -
Volatility :
Heart/Base -
Price Range :
€
Physico chemical properties
-
Appearance :
Colorless liquid -
Density :
1,054 -
Refractive Index @20°C :
Data not available. -
Optical rotation :
Data not available. -
Vapor pressure :
Data not available. -
Flash Point :
126°C
-
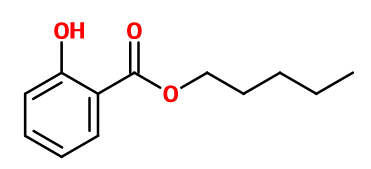
Molecular formula :
C12H16O3 -
Molecular Weight :
208,26 g/mol -
Log P :
>4,4 -
Fusion Point :
-12°C -
Boiling Point :
282°C -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Amyl Salicylate is used in white flowers, carnation, fougere, ambery and vanillic notes.
Year of discovery :
Initially marketed under the name Trefol, Amyl Salicylate was first used in the perfume Trèfle Incarnat - L.T. Pivert (1898) First synthesized in 1854 by reaction of salycile chloride with amyl alcohol by Drion et al.
Natural availability :
Amyl Salicylate is present in trace amounts in Osmanthus Absolute. It can therefore be extracted, but it is mostly the synthetic compound that is used in perfumery.
Isomerism :
PhenoxyEthyl IsoButyrate is a constitutional isomer of Amyl Salicylate. It smell is closer to rose than jasmine, and is not solar.
Synthesis precursor :
Amyl Salicylate is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Like other Salicylates, Amyl Salicylate is synthesized by an esterification reaction between salicylic acid and amyl alcohol (or pentenol). This reaction is catalysed by the presence of a strong concentrated acid such as sulfuric acid in the reaction medium.
Stability :
May form Salicylic acid through time.
Most of the time, the occurrence of a benzenic cycle in a molecule causes a coloration of this molecule through time.
Unstable in acidic products, except antiperspirants, and in bleach.
Other comments :
Isoamyl Salicylate has a more metallic and fruity aspect, while Amyl Salicylate has a pronounced jasmine note.
It is one of the most powerful salicylates.
Regulations & IFRA
Allergens :
This ingredient is classified as an allergen under European Regulation 2023/1545, dated August 26, 2023.
Its presence must therefore be declared on product labels when it exceeds 0.001% in leave-on products and 0.01% in rinse-off products.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

